Native recombinant human endoplasmic reticulum chaperones from yeast cells.
BACKGROUND
Endoplasmic reticulum (ER) chaperones are multifunctional proteins involved in a variety of biological processes such as protein folding and quality control in the ER, unfolded protein response, MHC class I antigen processing, as well as other important functions these proteins play outside of the ER. The role of ER chaperones in various human diseases seems to be especially important. In recent years there are growing amount of data demonstrating involvement of particular ER chaperones in many pathological processes. These recent findings suggest possible application of ER chaperones in therapeutic trials and development of new pharmaceuticals. There is also a wide field for other applications using chaperones in fundamental and applied studies. The recombinant protein expression technologies should be considered for efficient and safe production of these proteins.
PRODUCTS
We offer three main human ER chaperone proteins GRP78/BiP and GRP58/ERp57 that are produced in the yeast cells.
Our recombinant proteins are superior for use in various pharmaceutical applications for several reasons:
- Tag-free. No tags are used for purification of these recombinant proteins.
- Correctly processed in yeast cell and the final protein product is composed from exactly the same amino acid sequence as in human cells.
- Non-native modifications are not present in these recombinant products.
- Oligomeric state of these recombinant proteins corresponds to that of native human chaperone proteins isolated from human tissues.
Fully active. An example of ATPase activity of GRP78/BiP from yeast in comparison to E. coli-expressed human BiP is given below.
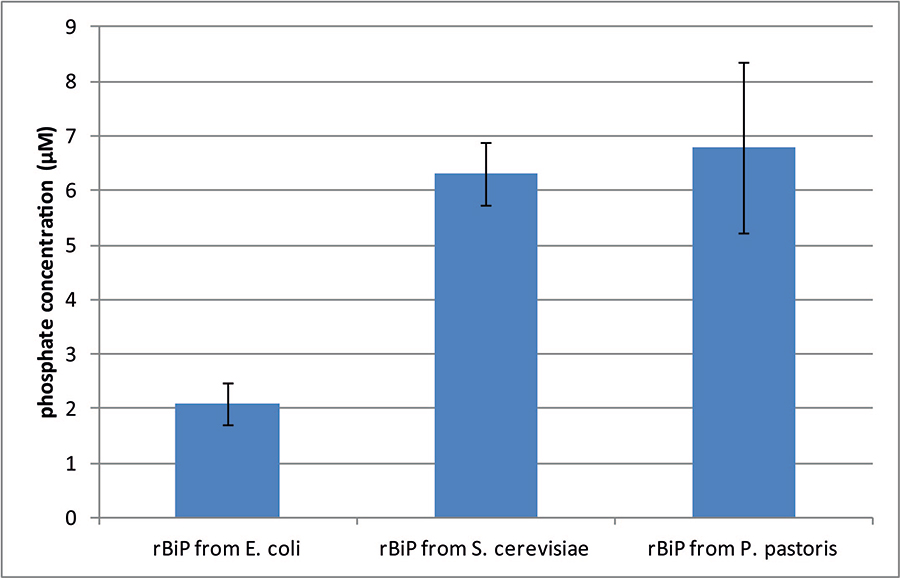
Figure. ATPase activity of yeast-secreted human BiP. The amount of released phosphate by 1 μg of either bacterial or yeast-derived human BiP was determined after incubation at 25°C for 75 min. with a non-radioactive procedure. Values are the mean of three separate experiments with an error bar representing SD.
(Note: detailed description of experimental procedure can be found in Čiplys et al., Microb Cell Fact. 2014 Feb 11;13:22.)
6. Stable.
These advantages makes those multifunctional recombinant products promising candidates for various applications.