Highly pure SARS-CoV-2 nucleoprotein from S. cerevisiae, frozen
Description: SARS-CoV-2 recombinant Nucleoprotein (SCoV2-rN) expressed in yeast Saccharomyces cerevisiae. UniProtKB sequence accession number: P0DTC9 (NCAP_SARS2), expressed with C-terminal His6-tag. It was purified from yeast lysates under native (non-denaturing) conditions using convenient chromatographic techniques to the high purity. Then it was dialyzed against PBS, adjusted to 1.0 mg/ml concentration, filter-sterilized, aliquoted and frozen for storage.
Form: Frozen liquid in PBS (pH 7.4) with 500 mM NaCl.
Safety: Product 20-S2N-ScB-F contains only yeast expressed proteins that are not infectious or hazard. Contains no plasma or serum of animal origin. No safety precautions are required.
Storage: The product is shipped with dry ice (shipment cost to Europe: 160 €). Store at -80°C for the long term. After thawing may be stored for short-term at 4°C (stable at least 1 week at this temperature). Avoid repeated freeze/thaw cycles.
Concentration: 1.0 mg/ml.
Image: SDS-PAGE showing SCoV2-rN product 20-S2N-ScB-F at approximately 47-48 kDa (3 μg/lane).
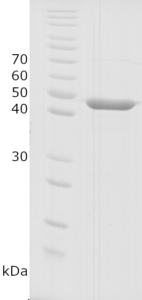
Purity: >97% pure SCoV2-rN protein as determined by SDS-PAGE.
Applications (tested): ELISA, other immunoassays, Total Internal Reflection Ellipsometry (TIRE), Spectroscopic Ellipsometry (SE), Quartz Crystal Microbalance with Dissipation (QCM-D), Electron microscopy, Western blot, SDS-PAGE.
Reactivity of SCoV2-rN with specific IgG in ELISA using Covid-19 positive and negative human sera: this product 20-S2N-ScB-F showed similar results in ELISA with COVID-19 positive and negative patient sera, as analogous protein construct of lower purity (see product 20-S2N-ScA-F information), but a lesser number of individual sera was tested.
Measured SCoV2-rN (product 20-S2N-ScB-F) interaction with a specific polyclonal antibody (anti-SCoV2-rN) by the total internal reflection ellipsometry (TIRE): association and dissociation rate constants (ka, kd), equilibrium association and dissociation constants (KA, KD) for SCoV2-N/anti-SCoV2-rN immune complex formation were determined (Ref. 1). These findings can be applied in the design of new antibody/antigen interaction-based analytical systems dedicated to the determination of anti-SCoV2-rN antibodies.
Measured SCoV2-rN (product 20-S2N-ScB-F) interaction with a specific polyclonal antibody (anti-SCoV2-rN) by the combined spectroscopic ellipsometry (SE) and quartz crystal microbalance with dissipation (QCM-D): The real-time frequency (ΔF) and energy dissipation (ΔD) change for affinity interaction of SCoV2-rN with anti-SCoV2-N were measured by SE/QCM-D (Ref. 2). The flexibility of the antibody Fab arms allows them to reach the more distantly located SCoV2-rN and to establish a bivalent interaction between proteins in formed SCoV2-N/anti-SCoV2-rN complex (Ref. 2).
Unit/Price:
| Size | Catalogue No. | Price | |
| 50 μg | 20-S2N-ScB-F-L | 150 € | Inquiry / Order product |
| 100 μg | 20-S2N-ScB-F-C | 225 € | |
| 500 μg | 20-S2N-ScB-F-D | 750 € | |
| 1 mg | 20-S2N-ScB-F-M | 1125 € |
References:
- Plikusiene I, Maciulis V, Ramanaviciene A, Balevicius Z, Buzavaite-Verteliene E, Ciplys E, Slibinskas R, Simanavicius M, Zvirbliene A, Ramanavicius A: Evaluation of kinetics and thermodynamics of interaction between immobilized SARS-CoV-2 nucleoprotein and specific antibodies by total internal reflection ellipsometry. J Colloid Interface Sci. 2021 Jul 15; 594:195-203. doi: 10.1016/j.jcis.2021.02.100. Full text
- Plikusiene I, Maciulis V, Juciute S, Ramanavicius A, Balevicius Z, Slibinskas R, Kucinskaite-Kodze I, Simanavicius M, Balevicius S, Ramanaviciene A: Investigation of SARS-CoV-2 nucleocapsid protein interaction with a specific antibody by combined spectroscopic ellipsometry and quartz crystal microbalance with dissipation. J Colloid Interface Sci. 2022 Nov 15; 626:113-122. doi: 10.1016/j.jcis.2022.06.119. Full text.