SARS-CoV-2 recombinant Spike (SCoV2-rS) glycoprotein trimeric ectodomain from mammalian CHO cells, in glycerol stock
Description: SARS-CoV-2 recombinant Spike (SCoV2-rS) glycoprotein ectodomain expressed as secreted trimeric protein in mammalian (hamster) CHO cells. UniProtKB sequence accession number: P0DTC2 (SPIKE_SARS2); expressed ectodomain includes amino acids (aa) 1-1208. Whole expression construct includes: full-length SCoV2-S ectodomain (aa 1 – 1208) w/o transmembrane and cytoplasmic aa, furin cleavage site “RRAR” mutated to “GSAS”, C-terminal GCN4 trimerization motif fused to protein sequence, then follows thrombin cleavage site, Strep-tag II and His6-tag. Two mutations (K986P and V987P) were introduced into SCoV2-S sequence to stabilize the trimer in the pre-fusion conformation. SCoV2-rS glycoprotein was purified from culture medium under native (non-denaturing) conditions using convenient chromatographic techniques. Final product is formulated in glycerol stock for long-term storage in liquid form at -20°C.
Form: formulated in glycerol stock solution (50%) in PBS (pH 7.4).
Storage: Shipped on dry ice (shipment costs: 160 € to Europe). Store at -20ºC in liquid form (solution does not freeze at this temperature).
Concentration: 0.5 mg/ml.
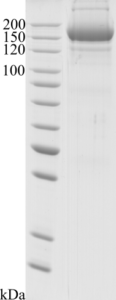
Purity: >90 % as determined by SDS-PAGE.
Image: SDS-PAGE showing SCoV2-rS product 20-S2S-TCg-G at approximately 160 kDa (3 μg/lane).
Applications (tested): ELISA, other immunoassays, Electron microscopy, Western blot, SDS-PAGE.
Quality: According to the expressed construct, purified SCoV2-rS protein should be trimer in the stabilized prefusion conformation. Full-length trimeric protein enables detection of more antibodies against SCoV2-S, than shorter protein constructs (e.g., RBD or S1). This product has been compared both with S1 recombinant protein in ELISA and S1 antigen-based CE-approved commercial IVD tests using the same set of human sera. Results for sensitivity and specificity are provided in the Table below:
| Reference test for Covid-19 positive sera |
RT-PCR |
|||
| Analyzed antigens or serological tests | SARS2 S IgG (Baltymas) | SARS2 S1 IgG (Sino Biological) |
AMP rapid IgG test | Euroimmun ELISA (S1) IgG test |
| Sensitivity (n=50), correct positives | 94.00% | 78.00% | 92.00% | 92.00% |
| Specificity (n=150 or n=160), correct negatives | 99.38% (n=160) |
97.33% (n=150) |
99.38% (n=160) |
98.75% (n=160) |
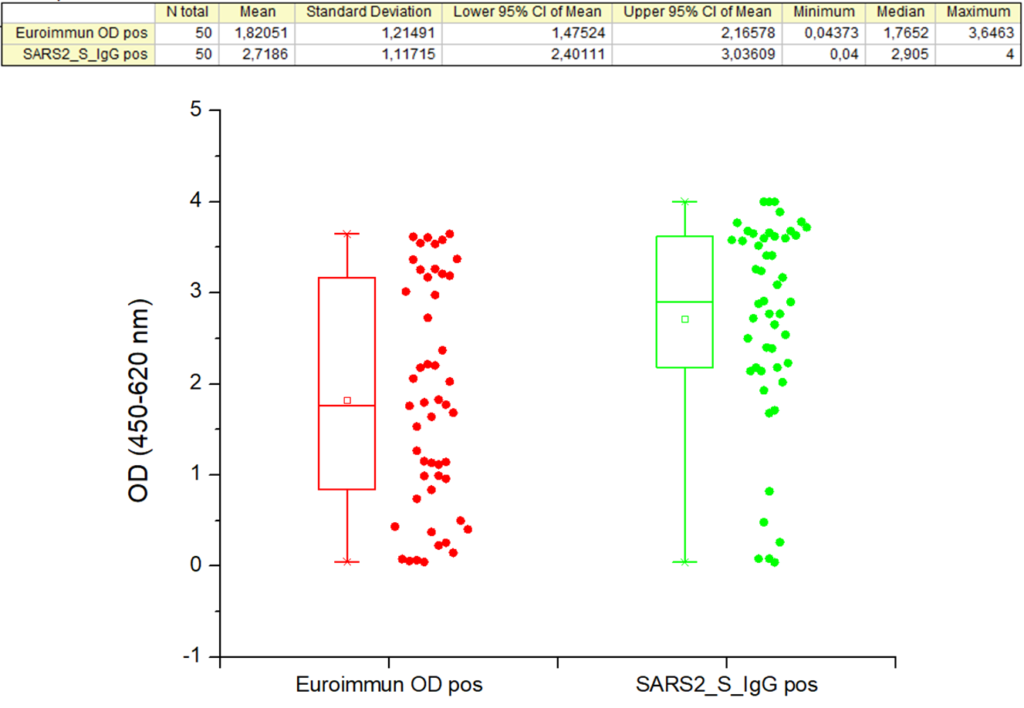
| OD mean for positive sera (n=50) | >2,718* | 0,980 | N. A. | 1,820 |
| Accuracy** | 99.11% | N. A. | 99.01% | 98.41% |
*The exact OD mean for Baltymas S protein is higher, as in three cases OD reached maximal value (4,000) of the equipment used.
** assuming a COVID prevalence of 5%, this indicates the likelihood that the test will correctly classify samples as positive and negative.
Comparison of Baltymas’s product 20-S2S-TCg-G (green) with EUROIMMUN AG (Germany) Anti-SARS-CoV-2 ELISA IgG (red) used as a “gold standard” (product No. EI 2606-9601 G; CE-marked and FDA-approved by EUA) in semi-quantitative IgG ELISA by testing 50 COVID-19 PCR-positive patient sera is shown below:
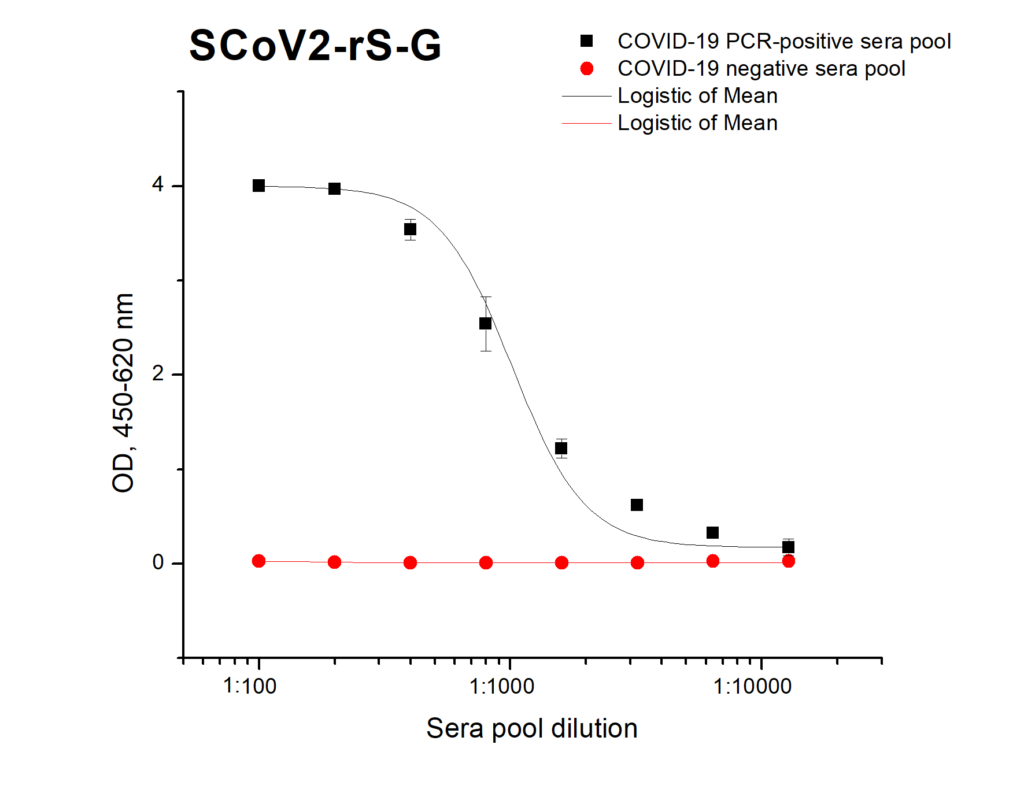
Reactivity of SCoV2-rS glycoprotein (current batch of product 20-S2S-TCg-G) with specific IgG in ELISA using pools of Covid-19 positive and negative sera:
Applications (approved): This SCoV2-rS product has been used in two CE-marked IVD tests, semi-quantitative and quantitative (in BAU/ml) IgG ELISAs.
Unit/Price:
| Size | Catalogue No. | Price | |
| 50 μg | 20-S2S-TCg-G-L | 325 € | Inquiry / Order product |
| 100 μg | 20-S2S-TCg-G-C | 500 € | |
| 500 μg | 20-S2S-TCg-G-D | 1875 € | |
| 1 mg | 20-S2S-TCg-G-M | 3125 € |
Note: This SCoV2-rS construct has also been used for scientific research that mostly included another formulation of the same glycoprotein (frozen in PBS). Please see the list of published articles in the product 20-S2S-TCg-F web page.