SARS-CoV-2 Spike glycoprotein Beta (B.1.351) variant trimeric ectodomain from mammalian CHO cells, frozen
Description: SARS-CoV-2 recombinant Spike Beta-SA (B.1.351) variant (SCoV2-rSβ) glycoprotein ectodomain expressed as secreted trimeric protein in mammalian (hamster) CHO cells. It contains ten mutations in comparison to the reference SARS-CoV-2 Spike protein sequence (UniProtKB accession number: P0DTC2 (SPIKE_SARS2)): L18F, D80A, D215G, LAL242-244del, R246I, K417N, E484K, N501Y, D614G, A701V; expressed ectodomain includes amino acids (aa) 1-1208. Whole expression construct includes: full-length SCoV2-S ectodomain (aa 1 – 1208) w/o transmembrane and cytoplasmic aa, furin cleavage site “RRAR” mutated to “GSAS”, C-terminal GSN4 trimerization motif fused to protein sequence, then follows thrombin cleavage site, Strep-tag II and His6-tag. Two mutations (K986P and V987P) were introduced into SCoV2-S sequence to stabilize the trimer in the pre-fusion conformation. SCoV2-rSβ glycoprotein was purified from culture medium under native (non-denaturing) conditions using convenient chromatographic techniques. Then it was dialyzed against PBS, adjusted to 1.0 mg/ml concentration, filter-sterilized, aliquoted, and frozen for storage.
Form: Frozen liquid in PBS (pH 7.4).
Storage: The product is shipped with dry ice (shipment cost to Europe: 160 €). Store at -80°C for the long term. After thawing may be stored for short-term at 4°C (stable at least 2 weeks at this temperature). Avoid repeated freeze/thaw cycles.
Concentration: 1.0 mg/ml.
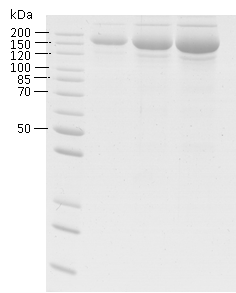
Purity: >90 % as determined by SDS-PAGE.
Image: SDS-PAGE showing SCoV2-rSβ product 21-S2SSA1-TCg-F at approximately 160 kDa (1, 3 and 5 μg/lane).
Applications (tested): ELISA, other immunoassays, Total Internal Reflection Ellipsometry (TIRE), other biochemical and biophysical analyses, Western blot, SDS-PAGE.
Quality: According to the expressed construct, purified SCoV2-rSβ protein should be trimer in the stabilized prefusion conformation. Full-length trimeric protein enables detection of more antibodies against SCoV2-S, than shorter protein constructs (e.g., RBD or S1). It provides a tool to develop highly sensitive classical immunoassays, as well as design novel physico-chemical methods for monitoring of interactions of SCoV2-S antigens with specific antibodies (see Reference). This product 21-S2SSA1-TCg-F showed similar results in ELISA with COVID-19 positive and negative patient sera, as initial sequence SCoV2-rS protein construct (see product 20-S2S-TCg-G information), but a lesser number of individual sera was tested. Besides, it was used for the development of the highly sensitive total internal reflection ellipsometry method for the evaluation of interaction kinetics between SCoV2-S and specific antibodies in human serum samples (Ref. 1).
Interaction kinetics measured between specific polyclonal human antibodies against SARS-CoV2 S protein (pAb-SCoV2-S) produced after vaccination with the Vaxzevria vaccine and SCoV2-rSα glycoprotein (product 21-S2SSA1-TCg-F) by total internal reflection ellipsometry (TIRE) method: association and dissociation rate constants, the equilibrium association (KA) and dissociation (KD) constants were calculated using a two-step irreversible binding mathematical model (Ref. 1). The results showed high-affinity binding of vaccine-induced pAb-SCoV2-S antibodies from human blood serum to the SCoV2-rS glycoprotein (Ref. 1). This enables monitoring of the humoral immune response by a highly sensitive, label-free, and real-time TIRE method after vaccination with COVID-19 vaccines.
Unit/Price:
| Size | Catalogue No. | Price | |
| 50 μg | 21-S2SSA1-TCg-F-L | 325 € | Inquiry / Order product |
| 100 μg | 21-S2SSA1-TCg-F-C | 500 € | |
| 500 μg | 21-S2SSA1-TCg-F-D | 1875 € | |
| 1 mg | 21-S2SSA1-TCg-F-M | 3125 € |
Note: Biotinylated form of this product is available under request.
Reference on the use of this product (21-S2SSA1-TCg-F):
1. Plikusiene I, Maciulis V, Juciute S, Maciuleviciene R, Balevicius S, Ramanavicius A, Ramanaviciene A: Investigation and Comparison of Specific Antibodies’ Affinity Interaction with SARS-CoV-2 Wild-Type, B.1.1.7, and B.1.351 Spike Protein by Total Internal Reflection Ellipsometry. Biosensors (Basel). 2022 May 18; 12(5):351. doi: 10.3390/bios12050351. Full text.